Optimization of the medicine clinical trials using intensive simulation
Analyze the possibilities of simulation use in the process related to the design of clinical trials, with the objective of reduce the time needed for evaluate new pharmaceutical products.
Publications related.
ASM 2005
http://www.iasted.org/conferences/pastinfo-469.html June 15 – 17, 2005 Benalmadena, (Spain) |
Date:
Monday, January 1, 2001 (All day) - Thursday, January 1, 2004 (All day)
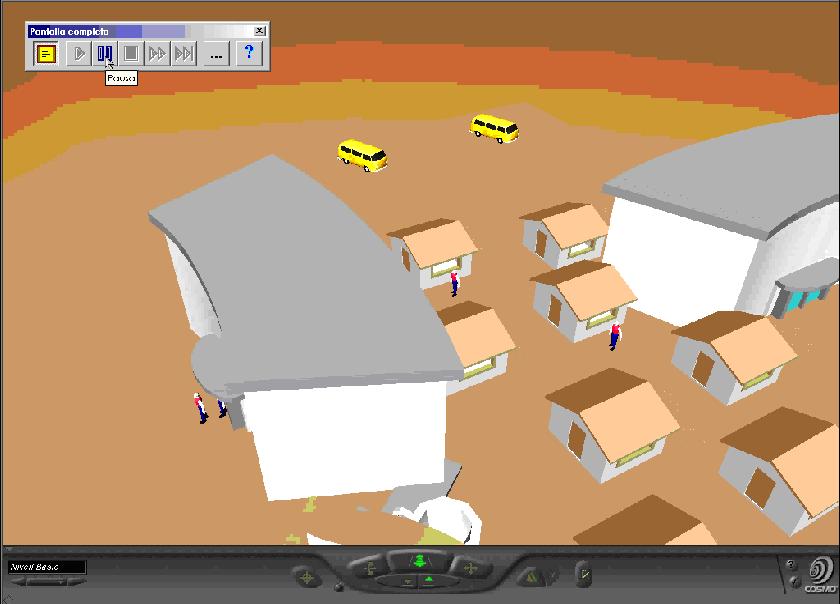
Clinical trials video
This video shows the Clinical trial simulation model.
|
This video shows the execution of the Clinical trial simulation model. The model was implemented using LeanSim system. |
ASM 2005 Presentation
Embedded Scribd iPaper - Requires Javascript and Flash Player
FACULTAT D’INFORMÀTICA DE BARCELONA
CLINICAL TRIAL SIMULATION WITH LEANSIM
Pau Fonseca i Casas Ismail Abbas Josep Casanovas LCFIB UPC
Clinical Trials
FACULTAT D’INFORMÀTICA DE BARCELONA
Expensive experiments. l Need long time until achievement of acceptable results. l Consider small or large sample size.
l
n n
This sample size many times is so small. Many clinical trials may fail to achieve their expected results. Also, errors committed at the time of the design, such as, wrong endpoint selection, can also leads to misestimate the sample size.
Clinical Trials models
FACULTAT D’INFORMÀTICA DE BARCELONA
l
Model construction is based on describing the process of the clinical trial to:
n
n
n
Evaluate the behavior of the model under different hypothetical parameters changes. To analyze the evolution of the selected respond parameter (in our case level of viral load in HIV patients) during the follow-up visits under the effect of both treatments. To evaluate the behaviour of the variables under study, from the initialization of the follow-up period of the trial until the evaluation of the last patient.
Clinical Trials conceptual model
Patient
FACULTAT D’INFORMÀTICA DE BARCELONA
Arrivals centers
1 2 3 4
/
New patient New center
5
Selection Inclusion exclusion /
Recruitment, inclusion, follow-up and economical sub-models of clinical trials
Treatment
Control treatment / new
1
2
3
4
5
Follow Statistical model Clinical model
- up
Terminal state End of trial Withdrawals Other causes
Epidemiological model
Economic model
Simulation of clinical trials
FACULTAT D’INFORMÀTICA DE BARCELONA
Cost design: Inclusion, follow-up Cost opportunity
Cost Sub-model
Recruitment sub-model
Inclusion sub-model
Follow-up sub-model
Total duration Total cost Statistical significance Power
Number of centres Arrivals/inclusion rates
Clinical-statistical model, epidemiologic model efficacy, sample size, test statistic, endpoints
LeanSim environment
FACULTAT D’INFORMÀTICA DE BARCELONA
l l
Event Scheduling simulation environment Have different elements:
n n
n n
LeanEditor, model’s edition tool. LeanGen implements event scheduling simulation engine. The engine owns its set of events enabling communications, via TCP/IP, with other simulation components. Lean Client shows model virtual representation. LeanStatistics allows data acquisition from a LeanSim model and shows the statistics in execution time.
FACULTAT D’INFORMÀTICA DE BARCELONA
LeanStatistics
LeanSim Clinical Trial construction
FACULTAT D’INFORMÀTICA DE BARCELONA
Patient inclusion
Visit N
No Is the last visit?
Yes Results
Model implementation
FACULTAT D’INFORMÀTICA DE BARCELONA
******************************************* ************* PROCESS.SCN **************** *** Fitxer de configuració de processos i entitats *** ******************************************* [ENTITATS] ENTITAT1=pacient [pacient:ATRIBUTS] FITXER=pacient.txt ATRIB1=Orientacion ATRIB2=Icon2D ATRIB3=Forma ATRIB4=Color ATRIB5=Escala [pacient] Orientacion_Info=6,Orientacion,0 Orientacion=0|0|0 Icon2D_Info=1,Icon2D,0 Icon2D=default.ico Forma_Info=2,Forma,0 Forma=Inici.wrl Color_Info=6,Color,0 Color=1|1|1 Escala_Info=6,Escala,0 Escala=1|1|1 [pacient:assaig] ACTION1=g(1),generar,12|Inclusio.vbs,NO,NO,pacient:assaig|0,1,pacient,12|Pacients.vbs; ACTION2=centre hospitalari(1),iterar,1|1|0.000000,NO,NO,; ACTION3=espera(1),esperar,12|TempsEspera.vbs,no,NO,; ACTION4=espera(1),goto,1|1|0,NO,NO,100|pacient:assaig|2; [pacient:final] ACTION1=final(1),destruir,1|1|1,No,NO,; [PROCESSOS] PROC1=pacient:final PROC2=pacient:assaig
Visual Basic modules
FACULTAT D’INFORMÀTICA DE BARCELONA
l
The visual basic script files that rule the model are:
n n n n
n
n
Condicio.vbs: Determines if patient finishes the experiment. Inclusio.vbs: Time between generations. TempsEspera.vbs: Time between two visits. Inicialitzacio.vbs: Contains initialization procedures for each patient. Iteracion.vbs: Determines operations done in each iteration (visit) in the center. Pacients.vbs: Determines the numbers of patients involved in the experiment.
l
Condicio.vbs is:
n
CurrentEntity.GetAttributeValue("visita") < 7
Inicialitzacio.vbs code
FACULTAT D’INFORMÀTICA DE BARCELONA
Dim b Dim edat Dim pes Dim sexe b = Simulator.GetUniform(0, 1) If (b < 0.72) then 'Men sexe = 0 CurrentEntity.SetAttributeValue "sexe", 0 (...) else 'Women sexe = 1 CurrentEntity.SetAttributeValue "sexe", 1 (...) end if (...) if (sexe = 0) then CurrentEntity.SetAttributeString "forma", "HomeBlau.wrl" else CurrentEntity.SetAttributeString "forma", "DonaBlau.wrl" end if else CurrentEntity.SetAttributeValue "tractament", 1 if (sexe = 0) then CurrentEntity.SetAttributeString "forma", "Home.wrl" else CurrentEntity.SetAttributeString "forma", "Dona.wrl" end if end if
FACULTAT D’INFORMÀTICA DE BARCELONA
Clinical trial LeanSim model
FACULTAT D’INFORMÀTICA DE BARCELONA
Results
FACULTAT D’INFORMÀTICA DE BARCELONA
Results
Conclusions
FACULTAT D’INFORMÀTICA DE BARCELONA
l Clinical
trials are always a large process that involves a lot of people and resources in a long period of time. l Duration of clinical trial determines cost and due to patients abandonment problem sometimes clinical trial is not valid. l Is difficult to compare different alternatives in order to determine what is the best approach.
Conclusions
FACULTAT D’INFORMÀTICA DE BARCELONA
l l
l
In this work a new clinical trials simulator is presented. Reproduction of clinical trial through simulation enables the possibility of determine if initial number of patients are enough for clinical trial typology, and compare different design alternatives. For pharmaceutical industry this tool can reduce clinical trial cost related to the launch of a new medicament, and for society the use of this tool represents faster use of new medical treatments.
Questions?
FACULTAT D’INFORMÀTICA DE BARCELONA
l l l l
Pau Fonseca i Casas
n
pau@fib.upc.edu esmail@fib.upc.edu josepk@fib.upc.edu
Ismail Abbas
n
Josep Casanovas
n
C/ Jordi Girona, 1-3 08034 Barcelona Phone:+34 93 401 70 00
FACULTAT D’INFORMÀTICA DE BARCELONA
CLINICAL TRIAL SIMULATION WITH LEANSIM
Pau Fonseca i Casas Ismail Abbas Josep Casanovas LCFIB UPC
Clinical Trials
FACULTAT D’INFORMÀTICA DE BARCELONA
Expensive experiments. l Need long time until achievement of acceptable results. l Consider small or large sample size.
l
n n
This sample size many times is so small. Many clinical trials may fail to achieve their expected results. Also, errors committed at the time of the design, such as, wrong endpoint selection, can also leads to misestimate the sample size.
Clinical Trials models
FACULTAT D’INFORMÀTICA DE BARCELONA
l
Model construction is based on describing the process of the clinical trial to:
n
n
n
Evaluate the behavior of the model under different hypothetical parameters changes. To analyze the evolution of the selected respond parameter (in our case level of viral load in HIV patients) during the follow-up visits under the effect of both treatments. To evaluate the behaviour of the variables under study, from the initialization of the follow-up period of the trial until the evaluation of the last patient.
Clinical Trials conceptual model
Patient
FACULTAT D’INFORMÀTICA DE BARCELONA
Arrivals centers
1 2 3 4
/
New patient New center
5
Selection Inclusion exclusion /
Recruitment, inclusion, follow-up and economical sub-models of clinical trials
Treatment
Control treatment / new
1
2
3
4
5
Follow Statistical model Clinical model
- up
Terminal state End of trial Withdrawals Other causes
Epidemiological model
Economic model
Simulation of clinical trials
FACULTAT D’INFORMÀTICA DE BARCELONA
Cost design: Inclusion, follow-up Cost opportunity
Cost Sub-model
Recruitment sub-model
Inclusion sub-model
Follow-up sub-model
Total duration Total cost Statistical significance Power
Number of centres Arrivals/inclusion rates
Clinical-statistical model, epidemiologic model efficacy, sample size, test statistic, endpoints
LeanSim environment
FACULTAT D’INFORMÀTICA DE BARCELONA
l l
Event Scheduling simulation environment Have different elements:
n n
n n
LeanEditor, model’s edition tool. LeanGen implements event scheduling simulation engine. The engine owns its set of events enabling communications, via TCP/IP, with other simulation components. Lean Client shows model virtual representation. LeanStatistics allows data acquisition from a LeanSim model and shows the statistics in execution time.
FACULTAT D’INFORMÀTICA DE BARCELONA
LeanStatistics
LeanSim Clinical Trial construction
FACULTAT D’INFORMÀTICA DE BARCELONA
Patient inclusion
Visit N
No Is the last visit?
Yes Results
Model implementation
FACULTAT D’INFORMÀTICA DE BARCELONA
******************************************* ************* PROCESS.SCN **************** *** Fitxer de configuració de processos i entitats *** ******************************************* [ENTITATS] ENTITAT1=pacient [pacient:ATRIBUTS] FITXER=pacient.txt ATRIB1=Orientacion ATRIB2=Icon2D ATRIB3=Forma ATRIB4=Color ATRIB5=Escala [pacient] Orientacion_Info=6,Orientacion,0 Orientacion=0|0|0 Icon2D_Info=1,Icon2D,0 Icon2D=default.ico Forma_Info=2,Forma,0 Forma=Inici.wrl Color_Info=6,Color,0 Color=1|1|1 Escala_Info=6,Escala,0 Escala=1|1|1 [pacient:assaig] ACTION1=g(1),generar,12|Inclusio.vbs,NO,NO,pacient:assaig|0,1,pacient,12|Pacients.vbs; ACTION2=centre hospitalari(1),iterar,1|1|0.000000,NO,NO,; ACTION3=espera(1),esperar,12|TempsEspera.vbs,no,NO,; ACTION4=espera(1),goto,1|1|0,NO,NO,100|pacient:assaig|2; [pacient:final] ACTION1=final(1),destruir,1|1|1,No,NO,; [PROCESSOS] PROC1=pacient:final PROC2=pacient:assaig
Visual Basic modules
FACULTAT D’INFORMÀTICA DE BARCELONA
l
The visual basic script files that rule the model are:
n n n n
n
n
Condicio.vbs: Determines if patient finishes the experiment. Inclusio.vbs: Time between generations. TempsEspera.vbs: Time between two visits. Inicialitzacio.vbs: Contains initialization procedures for each patient. Iteracion.vbs: Determines operations done in each iteration (visit) in the center. Pacients.vbs: Determines the numbers of patients involved in the experiment.
l
Condicio.vbs is:
n
CurrentEntity.GetAttributeValue("visita") < 7
Inicialitzacio.vbs code
FACULTAT D’INFORMÀTICA DE BARCELONA
Dim b Dim edat Dim pes Dim sexe b = Simulator.GetUniform(0, 1) If (b < 0.72) then 'Men sexe = 0 CurrentEntity.SetAttributeValue "sexe", 0 (...) else 'Women sexe = 1 CurrentEntity.SetAttributeValue "sexe", 1 (...) end if (...) if (sexe = 0) then CurrentEntity.SetAttributeString "forma", "HomeBlau.wrl" else CurrentEntity.SetAttributeString "forma", "DonaBlau.wrl" end if else CurrentEntity.SetAttributeValue "tractament", 1 if (sexe = 0) then CurrentEntity.SetAttributeString "forma", "Home.wrl" else CurrentEntity.SetAttributeString "forma", "Dona.wrl" end if end if
FACULTAT D’INFORMÀTICA DE BARCELONA
Clinical trial LeanSim model
FACULTAT D’INFORMÀTICA DE BARCELONA
Results
FACULTAT D’INFORMÀTICA DE BARCELONA
Results
Conclusions
FACULTAT D’INFORMÀTICA DE BARCELONA
l Clinical
trials are always a large process that involves a lot of people and resources in a long period of time. l Duration of clinical trial determines cost and due to patients abandonment problem sometimes clinical trial is not valid. l Is difficult to compare different alternatives in order to determine what is the best approach.
Conclusions
FACULTAT D’INFORMÀTICA DE BARCELONA
l l
l
In this work a new clinical trials simulator is presented. Reproduction of clinical trial through simulation enables the possibility of determine if initial number of patients are enough for clinical trial typology, and compare different design alternatives. For pharmaceutical industry this tool can reduce clinical trial cost related to the launch of a new medicament, and for society the use of this tool represents faster use of new medical treatments.
Questions?
FACULTAT D’INFORMÀTICA DE BARCELONA
l l l l
Pau Fonseca i Casas
n
pau@fib.upc.edu esmail@fib.upc.edu josepk@fib.upc.edu
Ismail Abbas
n
Josep Casanovas
n
C/ Jordi Girona, 1-3 08034 Barcelona Phone:+34 93 401 70 00
